Do I need to submit an IND?
Options for Determining Whether the FDA Would Require an IND for the Proposed Clinical Trial
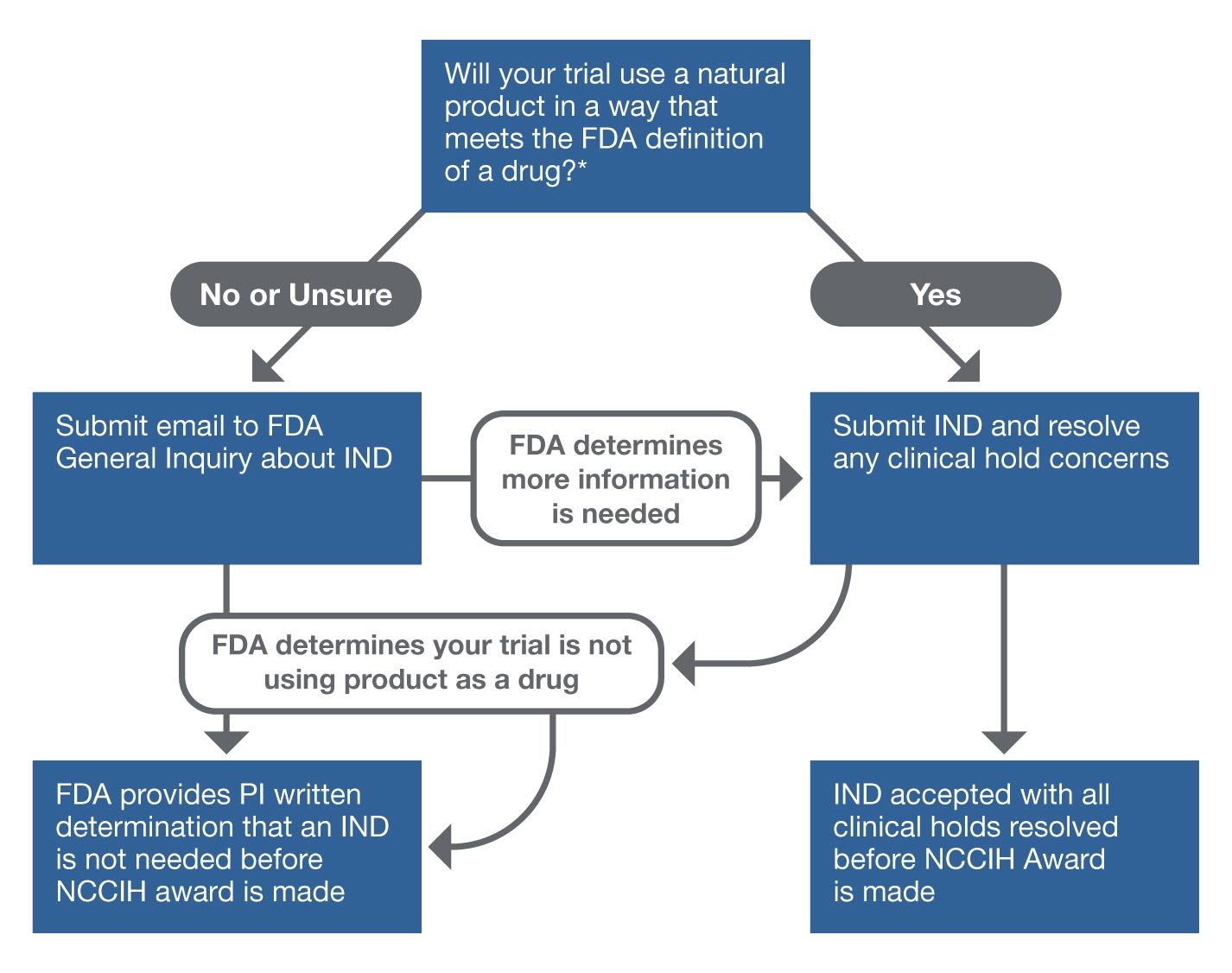
This figure provides an overview of the process for determining if the FDA will require an IND application for an investigator’s proposed research. For research in which human participants will ingest or apply a natural product, NCCIH requires either the IND application number for the proposed research or written documentation from the FDA that an IND application is not needed. The FDA must comment on an IND submission within 30 days. The FDA has no specific timeline for responses to general inquiry emails, but most investigators have reported reasonable timelines for replies.
* The FDA defines a drug, in part, as "a substance intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease."
Tips for Interacting with the FDA Regarding the IND Process
Contacting the FDA Through General Inquiry Email
The FDA has an email address dedicated to inquiries from researchers regarding the IND application process as it relates to dietary supplements. This email address can also be used to submit questions about the need to submit an IND application for a particular study design.
Submit FDA inquiries to: INDsFoodsDietarySuppCosmetics@fda.hhs.gov
What To Include in Your FDA General Inquiry Email
When inquiring about the need for an IND application, it is important to include all the relevant details about your study. Your email should include the following:
- The investigator’s name, complete mailing address, telephone number, email address, and organizational affiliation
- The title of the study protocol
- The name and a brief description, source (e.g., animal, synthetic), dosage form, trade name (if applicable), and supplier or manufacturer of each substance to be administered
- A statement about whether the study participants will receive the marketed product in its unmodified form or as a special preparation
- A brief summary of the study, including the purpose, hypothesis, number of participants, participant population, and condition or disease (if applicable); the dose, route, and duration of substance administration; and the endpoint measures
- A copy of the protocol (if available)
- A brief explanation of why you consider the substance safe for administration to human participants under the conditions of the study (include references, if possible)
- Justification and supporting data if doses greater than the labeled doses for dietary supplements will be used (include references, if necessary)
- A statement indicating whether you will charge participants for the product.