MBI Building Blocks and Common Data Elements

The essential components of a music-based intervention (MBI) include the intervention itself, the mode of delivery, the target population, a study design that distinguishes the MBI’s effects from those of socialization during the experience, and data collection and management. Figure 2 provides examples of various building blocks that should be incorporated in the planning and implementation of MBI studies.
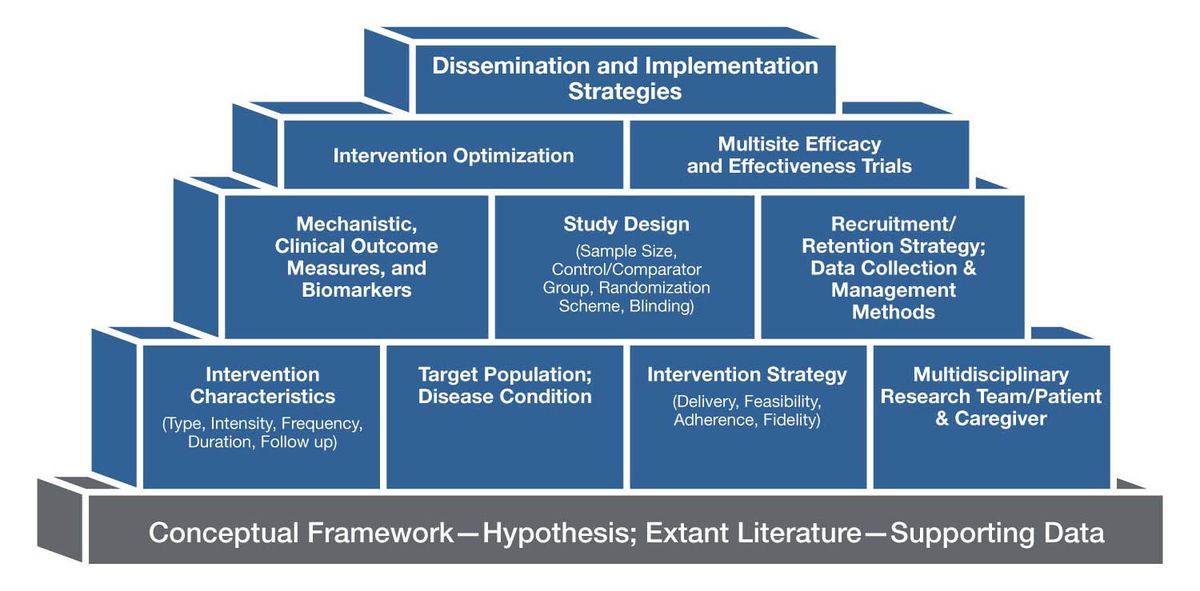
Figure 2. Examples of Building Blocks for Music-Based Interventions
Intervention
When defining the intervention, investigators should consider specific elements of music (e.g., pitch, timbre, rhythm), modes of engagement such as actively performing and creating music or passively listening to music, the relationship between culturally specific music preferences and outcomes, patient demographics, and contextual factors. In the earliest stage of the research, parameters such as dose and frequency should be treated as experimental variables to be manipulated to enhance efficacy. Furthermore, longer, lower intensity interventions and/or the inclusion of “booster” sessions may be important to maintain the desired treatment response. In this toolkit, we define the building blocks of the MBI in the context of a research setting—the rationale, clinical population, and aim of the study will be determined by the research question.
Protocol Delivery
A systematic review of music intervention trials reported that less than half of those studies examined treatment fidelity[23] (i.e., the extent to which an intervention is implemented consistently across practitioners and trial sites). Similar findings were noted in the 2020 Agency for Healthcare Research and Quality/National Academies of Sciences, Engineering, and Medicine report.[50] Critical considerations about the delivery methods for MBI studies include: (1) music characteristics (e.g., live versus recorded, music genre, personalized versus non-personalized; patient-selected versus investigator-selected); (2) research setting (e.g., laboratory, clinic, community); (3) mode of participation (e.g., individual versus group, in-person versus remote delivery); (4) timescale (e.g., short- versus long-term, session length and intensity, follow-up frequency, potential for habituation); (5) staffing and support (e.g., expert therapists versus wide range of providers with appropriate training); (6) resources and organization (e.g., cost, scheduling, assessments); and (7) feasibility, accessibility, and adherence. These considerations are equally important to objectively establish the intensity of the intervention needed to observe clinical improvements and maximize dissemination and implementation of the intervention.[21]
Study Population
The study population is the subset of the target population available for a study (e.g., individuals diagnosed with early onset dementia from a group of nursing home residents). MBI investigators must have a strong rationale for testing a specific intervention in any given population, for example, the choice of the target population might focus on disorder subtypes or severity, ability to conduct convenience sampling in a pilot study, availability of a control group in a randomized trial, or the relative importance of studying time courses longitudinally. Additional pragmatic considerations include accessibility to the population, its stability over time, and the resources available in the setting where the intervention will be tested. Additionally, screening of patients to eliminate confounding variables, such as hearing loss or amusia, is advisable.[51]
Control Groups or Comparators
Although control groups or comparators may not always be needed for early-phase research, they are crucial for randomized controlled trials. The goals of a study and the research question should drive the selection of the comparator group. Devising the appropriate control condition for music studies is complex and current MBI research has poorly designed control conditions. For MBI studies, control conditions may include a music element such as a slowed-down version of the same music, meaningless sounds or nature sounds, or, instead of a music element, the control may be an audiobook. The investigative team needs to consider whether the chosen control condition is intended to control for intervention effects that are unrelated to the music (e.g., attention, sound stimulation, visual stimulation, shared experience) or for effects of the specific intervention protocol delivery method (e.g., recorded music listening versus guided or tailored music listening). Moreover, control conditions must match the test intervention in intensity of engagement and observation (controlling for the Hawthorne effect). Equally important is controlling for possible placebo effect by matching the test intervention in the intensity of the treatment delivered.
The optimal comparator is one that will provide the clearest answer to the primary research question or the strongest test of the trial's primary hypothesis. The rationale for the comparator choice should focus on the primary purpose of the trial and not be weakened by lesser considerations or arbitrary rules. An NIH expert panel issued a useful framework for considering and justifying control groups with the Pragmatic Model for Comparator Selection in Health-Related Behavioral Trials.[52]